Table of Contents
Atoms form the foundation of chemistry and everything else that exists in the universe. Every matter is composed of densely packed atoms.
John Dalton proposed the first modern atomic model or can be said as discovered atoms. The physicist J.J.Thomson discovered the electrons in the year 1897. English physicist James Chadwick discovered neutron in the year 1932. Ernest Rutherford discovered protons in the year 1909.
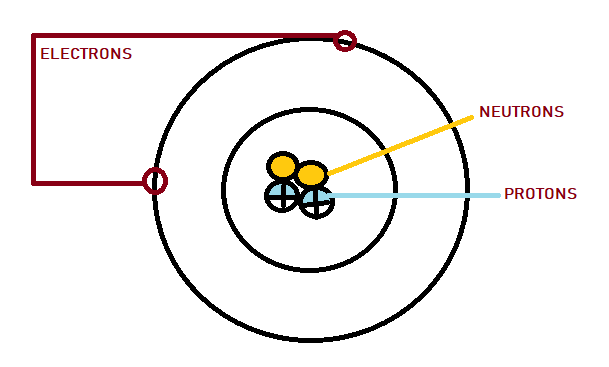
Each atom consists of small particles: protons, neutrons and electrons. Electrons are the smallest pieces among the three particles that make up an atom. Electrons have negative charges. Protons have positive charges.
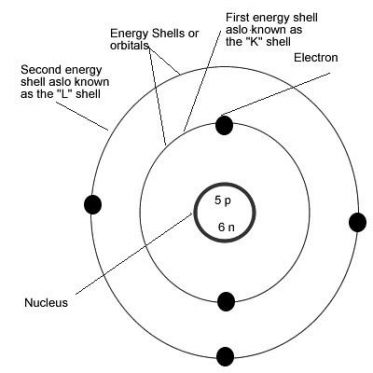
The protons and neutrons are present in the nucleus of the atom and the elcetrons rotate around in different orbits. These orbits are known by different alphabet names
The closest orbit to nucleus– K Shell
The second closest orbit to nucleus—L Shell
For more information on orbital shells , one can refer to
https://van.physics.illinois.edu/qa/listing.php?id=1224&t=atoms-shells-and-orbitals
https://education.jlab.org/qa/historyele_02.html
https://chem.libretexts.org/Bookshelves/…Atoms/Quantum_Numbers
Today, we will learn the intricate properties of these three subatomic particles electrons, protons and neutrons. Here, we discuss the atomic mass, relative atomic weight and several other aspects of atoms.
Atomic weight and atomic mass are the two most vital concepts you come across in Physics as well as in Chemistry. Most students assume that both the terms are interchangeable but they are not. Atomic mass is the total number of protons and neutrons in an atom or isotope.
Atomic weight is the mass of all the naturally occurring isotopes present in an element. Atomic weight is also known as the relative mass of an atom. Both of them are expressed in the unit called (amu) or atomic mass unit.
A single atom mass (ma) is the collection of the masses of all three subatomic particles present in it: protons, neutrons and electrons.
The relative mass is expressed as a multiple of one-twelfth of the mass of a carbon 12 atom. This means that the relative atomic mass denotes how many times an average atom of an element is heavier than one-twelfth of an atom of carbon 12.
What is the relative mass of a proton?
The relative mass of Proton = 1.6726231*10-27 kg
What is the relative mass of a neutron?
Neutron = 1.6749286*10-27 kg
What is the relative mass of an electron?
Electron = 9.1093897*10-31 kg
The relative mass of protons and neutrons are considered as “1’to avoid complicacy while solving the mathematical problems.
Relative mass of an electron is one of the fundamental constants of Physics. The relative mass of an electron is also known as “rest mass” (the mass of a stationary electron needs to be calculated to determine its value). For mathematical derivations, the mass is considered to be zero in most cases.
In absolute units, protons and neutrons have the mass of 10-27 kilograms. Electrons have an even smaller mass as compared to a proton which is 10-30.
The standard value of atomic weight or relative atomic massis derived from the value that is cited for that element in the periodic table
Protons, neutrons and electrons are the fundamental subatomic particles of an element.
Dalton’s Atomic Theory discusses the first building block of the concepts on matter and its compositions. However, it was not totally accurate. Years of scientific investigation finally revealed that atoms could be broken into even smaller subunits or subatomic particles. When Rutherford proposed that electrons orbit a positive nucleus, scientific research found a completely new dimension to the study.
In later experiments, Rutherford found a smaller positively charged particle in the nucleus and named it proton. He had also proposed the presence of a neutral particle, within the nucleus. This neutral particle was the ‘neutron’ (which was confirmed after a series of experiments, conducted by his student James Chadwick).
Protons and neutron are heavier elements that reside at the centre of the atom within the nucleus. The extremely lightweight particle electron, exist in a cloud orbiting the nucleus.
Protons are positively charged particles that exist within the atomic nuclei.
Some facts about protons
The number of protons in an atom is also the same as the atomic number of that element.
The atomic number can be thus defined as the total number of protons present in an element.
The electrons are negatively charged elements that are electrically attracted to the positively charged protons. J.J. Thomson, a British physicist, discovered electron and initially named it “corpuscles.”
Some facts about electron
Electron configuration of an atom help determine the stability, conductivity and boiling points of atoms of elements.
These particles constitute every atomic nucleus except for ordinary hydrogen. It was discovered by James Chadwick in 1932. Neutrons are particles of an atom that has a neutral charge. Neutrons are neither positively charged (like protons) nor negatively charged (like electrons).
Some facts about neutrons
Neutrons play a major role in mass and radioactive properties of an atom. Within a few years of its discovery, it was revealed that various elements undergo a type of nuclear reaction (fission) when bombarded by neutrons. The nucleus of a heavy element split into two nearly equal halves in case of each nuclear reaction that can continue as a chain reaction.
In 1942, Enrico Fermi and a group of American researchers demonstrated how the chain reaction of the fission process works. In subsequent years, this development led to the foundation of the atomic bomb.
Neutrons undergo radioactive decay where it breaks down into a proton, an electron and an antineutrino. This is also known as beta decay and the half-life for this decay is 614 seconds. A neutron has a relative mass of 1 and has a physical mass of 1.6749x10-27 kg.
Atoms which are almost same but have different number of neutrons are called isotopes.
Atoms with too many neutrons in their nucleus, renders them unstable. They’re called radioactive.
Atoms with extra electrons or missing electrons are referred as ions. They can have either a positive charge or a negative electric charge.
Every atomic particle has a twin anti-particle, with an opposite electric charge. The oppositely charged twin atoms are called antimatter.
According to Physics, the proton-to-electron mass ratio (μ or β) is considered as the rest mass of the proton divided by that of the electron. As this is a ratio of similar dimensioned physical quantities, the resultant becomes dimensionless. The final numerical values are independent of the system of units as well.
μ = mp/me= 1836.15267389(17)
The enclosed parenthesis is the “measurement uncertainty” where the value of μ is about 0.1 parts per billion.
Do you want to learn about matter and its intricate components in detail? Talk to your professor or seek help from the Physics assignment experts and boost your grades this semester.
Do you need help with complicated chapters like metallurgy, atomic structure, inorganic and organic chemistry or properties of carbon? The experienced PhD scholars and proficient writers of MyAssignmenthelp will guide you in every way. They will not only write your assignments but provide you with excellent study materials on the following areas:
We have hired some of the excellent writers who have completed their doctorates and hold the highest degree in the discipline. Hire our experts to put an end to all your academic worries and soar the sky, with top academic grades.
Suggested Reads:-
https://www.wikilectures.eu/w/Atomic_Models
https://sciencing.com › Science › Chemistry › Atomic & Molecular Structure
https://www.nature.com/articles/nature08879