Table of Contents
Electrical conductivity is a measurement of a material’s electrical current carrying capacity or conductivity. The term “specific conductance” also applies to electrical conductivity. An inherent quality of a material is conductivity.
The SI unit for electrical conductivity is siemens per metre (S/m), which is represented by the symbol. The Greek letter is utilised in the field of electrical engineering. Conductivity is sometimes represented by the Greek letter. Specific conductance, a measurement in comparison to that of pure water at 25°C, is frequently used to describe conductivity in water.
The reciprocal of electrical resistivity is electrical conductivity ():
σ = 1/ρ where for a material with a homogenous cross section, resistivity is:
The value = RA/l
Here, l is the material’s length, A is its cross-sectional area, and R is its electrical resistance.
As the temperature is decreased in a metallic conductor, electrical conductivity steadily increases. A loop of superconducting wire could conduct an electrical current without the need for external power below a threshold temperature since superconductors’ resistance falls to zero at this temperature.
Different materials conduct electricity through the movement of either electrons or holes in their bands. In electrolytes, ions carry the electric charge while moving around. The number of ionic species present in a material’s electrolyte solution greatly affects its conductivity.
Examples of substances with a high electrical conductivity include metals and plasma. Silver, a metal, is the element that is the best electrical conductor. Poor electrical conductivity is a property of electrical insulators like glass and pure water. On the periodic table, the majority of the nonmetals are poor thermal and electrical conductors. Semiconductors have conductivities that fall in between those of insulators and conductors.
Excellent conductors include the following:
Silver, Copper, Gold, Aluminium, Zinc, Nickel, Brass
Before you learn about why metal conducts electricity and how, there are few things that you need to know. They are –
Layers of organised atoms make up metals. A crystalline, three-dimensional structure is created by these atoms. That indicates that the atomic units that make up the solid are arranged in a manner that repeats itself on a regular basis.
Consider an atom cloud that lines up neatly. Each atom in the second line is right behind an atom in the first line, which is formed by another group. Along the same lines, a third row emerges. Atoms are arranged in tidy rows and columns in the resulting layer. When you gaze from front to back and from right to left, the pattern of atoms repeatedly repeats.
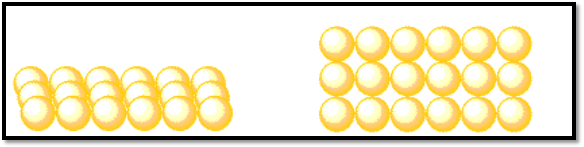
To begin, let’s use a neat and organized row of atoms as starting point. However, this time don’t place the second row of atoms directly behind the first row as they form. Instead, position them in between the atoms in the first row. Each atom in the second row will be placed so that it appears to be peering between two atoms in the first row.
There should be a small distance between the third row and the one in front of it. If you take a closer look, you’ll see that each atom in the third row is positioned one additional row behind an atom in the first row. From there, the fourth row will be slightly displaced, and so on.
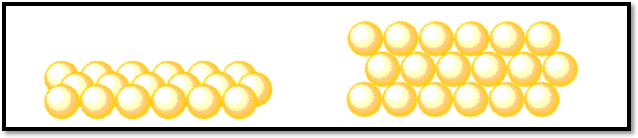
Circles do not jam up against one another without any room in between them like certain other geometric forms do. Holes are the spaces between atoms that make up a layer. Keep in mind that the word “holes” used here has nothing to do with conductivity-speak holes. Holes are simply the spaces between the atoms on this page. Holes in conductivity are regions with positive charge that could attract an electron.
The simplest unit in a pattern, also known as the unit cell, repeats itself. To cover every part of a layer, the unit cell is traced over and over again on the layer. The unit cell can be a single atom since each layer consists of unique atoms, which are spatially connected. To show the connection between atoms in a layer, we use the unit cell and often shift it to display multiple atoms.

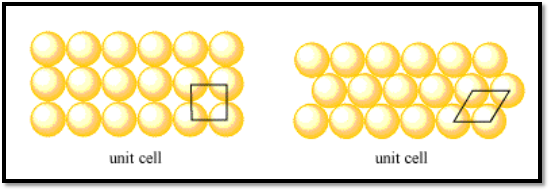
One of the things people are curious about in crystal structures is packing efficiency. It has to do with how closely a structure’s atoms are arranged. Frequently, the better the structure, the more densely packed it is
Because the atoms that make up these metals may be imagined as identical perfectly spherical, pure metals’ structures are simple to explain. Simple cubic (SC), body-cantered cubic (BCC), hexagonal closest-packed (HCP), and cubic closest-packed (CCP) are the four basic crystal forms that these substances all exhibit.
The valence shell of a metal typically contains 1, 2, or 3 electrons. As a result, they can easily give electrons. The reactivity factor of metal increases with the number of shells and decreases with the number of valence electrons.
When positively charged metal ions share a large number of valence electrons, it creates a “metallic bond.” This type of chemical bonding is responsible for the shiny appearance, malleability, and ability to conduct heat and electricity that metals possess.
When a metal is heated to the point of melting, the metallic bonds are not broken. Instead, these bonds are broken, resulting in the liquidation of the ordered array of metal ions and loss of their distinct, solid structure. When the metal is heated to the boiling point, these connections are entirely severed.
Drude and Lorentz proposed the classical free electron hypothesis in 1900. According to this idea, metals with unbound electrons abide by the principles of classical mechanics.
Classical free electron theory’s presumptions
There are 6 factors influencing the conductivity in metals
A metal’s atomic structure has a significant role in determining how well it conducts electricity. Metals are made up of atoms that are bound together by a sea of free-moving delocalized electrons that are responsible for the metal’s electrical conductivity. A metal will conduct electricity more efficiently the more delocalized electrons it possesses.
For instance, copper is a good conductor of electricity because it possesses a large amount of delocalized electrons. As a result, it serves as an electrical conductor.
The temperature of a metal can affect its electrical conductivity. As the temperature of a metal increases, the atoms and electrons within it become more mobile, which can lead to an increase in electrical conductivity. However, the relationship between temperature and electrical conductivity is not always straightforward. While some metals, such as copper and silver, maintain consistent electrical conductivities across a range of temperatures, others, like iron and aluminium, may become less conductive as the temperature increases.
The electrical conductivity of a metal can also be influenced by impurities or alien atoms. A metal loses some of its electrical conductivity when an impurity is added because it can obstruct the movement of delocalized electrons.
For instance, pure gold is a superb electrical conductor, but even minor levels of impurities, like copper or silver, can drastically reduce the conductivity of the metal. Similar to this, copper’s electrical conductivity can be decreased by the presence of impurities.
Doping is the procedure of adding impurities to a substance in order to change its electrical characteristics. For instance, semiconductors, which have intermediate electrical conductivity relative to metals and insulators, can be made from silicon by doping it with impurities like boron or phosphorus.
Stretching or compressing a metal’s lattice structure, also known as mechanical strain, can affect its electrical conductivity. This is because the distribution of delocalized electrons may change due to the strain.
Pressure can also have an impact on a metal’s electrical conductivity since a metal’s higher density can result in more delocalized electrons.
Particles that are electrically charged migrate, which results in electrical conductivity in metals. The existence of valence electrons, which are electrons in an atom’s outer shell that are free to move, is what distinguishes the atoms of metal elements. The ability of metals to conduct an electric current is due to these “free electrons”.
Valence electrons in a metal are able to move freely through the lattice structure, allowing them to carry an electric charge as they pass through the metal. This movement is similar to billiard balls colliding with each other in an electric field.
When resistance is low, energy transfer is at its highest. This is evident in a game of pool, where a ball collides with another and most of the energy is transferred to the next ball. As a result, each ball that is hit by a single ball will only receive a small amount of energy.
Similarly, metals with a single valence electron that is free to move and strongly repels other electrons are the most effective conductors of electricity. Copper, gold, and silver are the most conductive metals because they possess this property. Each metal has one valence electron that can move easily and generates a powerful repelling reaction.
The valence electron count is higher in semiconductor metals (or metalloids) and is typically four or higher. Thus, although having the ability to conduct electricity, they are ineffective at doing so. However, semiconductors like silicon and germanium can transform into incredibly effective electrical conductors when heated or doped with additional elements.
Ohm’s Law, which stipulates that the current is directly proportional to the electric field supplied to the metal, must be followed for conduction in metals. The law, which bears the name of German physicist Georg Ohm, first appeared in a paper published in 1827 that described how electrical circuits are used to measure current and voltage. The resistivity of a metal is the main factor to consider when using Ohm’s Law.
Electrical conductivity is the reverse of resistivity, which measures how effectively a metal resists the flow of current. This is typically measured as an ohm metre (m) across the opposing faces of a one-meter cube of material. Greek letter rho () is frequently used to indicate resistance.
On the other hand, electrical conductivity is typically expressed by the Greek symbol sigma () and measured in siemens per metre (Sm1). The reciprocal of one ohm is equivalent to one siemens.
Conductivity testing is used widely in many industries’ quality assurance and production procedures. For instance, certain businesses might use it to confirm the legitimacy of metals like aluminium, copper, or gold. Achieving the intended performance and longevity of the finished product depends on determining a material’s purity, strength, and integrity.
Organisations in the biological sciences, food and beverage, aviation, aerospace, pharmaceutical, electricity, water, and other industries are all affected by the measurement of electrical conductivity. Galvanised copper, for instance, is frequently used as a material in the production of printed circuit boards (PCBs) because of its high conductivity. However, producers must make sure the copper they use is pure and allows enough energy to flow through for best performance in order to ensure the circuit boards work properly.
Similar to this, the conductivity of parts used to build aircraft is assessed to determine their capacity for discharge, ensuring their ability to withstand material stress brought on by an event like a lightning strike.
Ans.Metals are able to conduct electricity because their electrons are free to move around. When electricity flows through a metal, the electrons help to transmit and distribute the electrical energy throughout the material. This is possible because of the mobility of the electrons within the metal.
Ans.Metals are excellent conductors of electricity and heat due to the formation of a matrix of atoms that allows outside electrons to travel easily. Instead of orbiting individual atoms, the electrons form a sea around the positively charged nuclei of the metal ions. Almost all metals have some degree of electrical conductivity.
Ans.Ions in ionic compounds conduct electricity. Ions cannot freely move about inside a solid because of the strong electrostatic forces that hold them together. Therefore, the ions are free to travel and can conduct electricity when they are liquid or in solution form.
Ans.Because it is simpler to shed valance electrons and generate cations, metals have a lower chance of gaining electrons. Metals’ nuclei do not exert a strong pull on their valence electrons, making it simpler for them to shed them. Therefore, it is well known that metals have lower electron affinities.
Ans.To achieve a fully stable octet, metals prefer to shed valence electrons in the form of cations. To lose electrons, they undergo endothermic energy absorption. Metals have a lower electron affinity than nonmetals. Nonmetals: In order to have a fully stable octet, nonmetals prefer to gain electrons to form anions.
Ans.Valence electrons are shared among metals, however they are not localised between specific atoms. Instead, they are entirely delocalized and dispersed throughout the metal. They are frequently depicted as a free-flowing “sea” of electrons that surround the atoms.