Describe FTIR and Raman spectroscopy. Explain why both the techniques are preferred to other characterisation techniques for analysis of Biomaterials. You are provided four different spectra. Interpret the spectra by identifying the key spectral peaks and describe in detail, how you have reached your conclusion in identifying the materials from the unknown spectra.
The endeavours of every absorption spectroscopy like ultraviolet-visible ("UV-Vis") spectroscopy, FTIR, etc. is to determine how efficiently a sample absorbs light at every wavelength (FTIR Applied to Biological Systems, 2007). The most common is the "Dispersive Spectroscopy" technique, this is to gleam a monochromatic light in the form of beams at a sample, then measure the amount of the light absorbed, and repeat this technique for different wavelength.
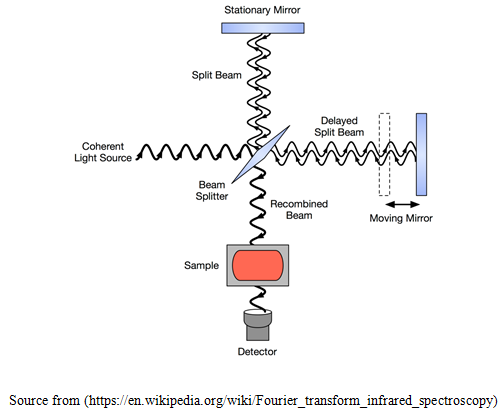
Fourier transform (FTIR) spectroscopy is a less spontaneous way to get the same information. Instead of falling on a monochromatic beam at the sample, this technique falls a beam containing numerous number of frequencies of light at the same time, and measures the amount of the beam that is absorbed by the sample (Berthomieu and Hienerwadel, 2009). After that, the beam is changed and modified which have a different combination of frequencies, giving a second observation data. This process re-occurrs many times. Later, a computer compares all these data and conclude the value of absorption at each wavelength. The beam explained above is produced by initiating with a light source of broadband that is the one consists of the full spectrum of different wavelengths to be measured. The light falls into a Michelson interferometer, that comprises of a mirror and its movement is controlled by a motor (Sánchez-de-la-Llave, 2009). Since this mirror moves, every beam of light with different wavelength cyclically blocked and transmitted, then again blocked and transmitted, because of wave interference, by the interferometer. Contrasting wavelengths are amended at different rates, to get the different spectrum of each beam that is emitted out of the interferometer. Computer processing is obligatory to get the desired result (light absorbed at each wavelength) from the raw data (light absorbed at each position of the mirror). The processing needs to be turned out to a common algorithm known as the Fourier transform hence it is named "Fourier transform spectroscopy". The unprocessed or raw data are at times called an "interferogram" (Hanley, 2012).
FTIR spectroscopy gives alike, but opposite, information (Infrared Spectroscopy, 1968). FTIR spectra tells the composition of gases, liquids and solids. The most frequent use is in the detection of unknown sample and evidence of manufacture sample (outgoing or incoming). The information material is very precise in most of the cases, explaining fine differences between like materials or sample. FTIR is less time consuming and this property makes it useful, particularly in screening applications. The entire scope of FTIR applications is wide-ranging.
Clinical applications of FTIR
Clinical applications of FTIR include the Deformulation of rubbers, polymers, etc., throughout (TGA-IR) thermogravimetric infra-red or (GC-IR) gas chromatography infra-red examination, Quality confirmation of outgoing / incoming samples, Analysis of coatings and thin films, supervising the automotive emissions (NORRISH, 1960). FTIR is highly sensitive and fast method to attain spectrum of high quality, this spectroscopy provides superior signal to noise ratio as contrast to the other dispersive instrument, spectrum can be attained very rapidly and saves time, gases, liquids as well as solids can be examined with FTIR, no external calibration is needed and provides accurate results. It is a non-destructive spectroscopic technique, Inorganic compounds as well as organic compounds can be identified effortlessly using this technique, Mechanical breakdown is almost nil in this spectroscopy as compared to others as a mirror is the only part moving in FTIR. Instantaneous analysis can be made for various gaseous compounds, FTIR can recognize even contaminants with small concentrations, it generally takes about 1 to 2 seconds for scanning, with High resolution.
After Sir C. V. Raman, Raman spectroscopy is named. It is a technique of spectroscopy that is used to examine the vibrational, rotational, and other modes with low-frequency in a system (Raman Spectroscopy, 2006). Raman spectroscopy is usually used in chemistry to give a fingerprint of molecules for its identification. It is based on Raman scattering, or inelastic scattering, generally from a laser in the visible, near UV, or near IR range, of monochromatic light.
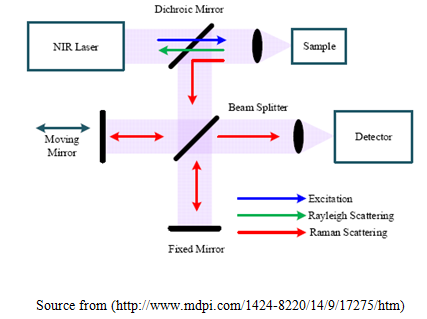
The laser interacts with photons, molecular vibrations or other excitations in the system, which results in the shifting that is up or down on energy of the laser. The vibrational movement in the system can be identified by the shift in energy. Normally, a sample is elucidated through a laser beam. From the illuminated speck Electromagnetic emission is gathered by means of a lens and transmitted all the way through a monochromatic. Expandable scattered rays at the wavelength equivalent to the laser column (Rayleigh scattering) is passed through a filter, whereas the rest of the accumulated light is disseminated on top of a detector by either a band pass filter or a notch filter (Chukov, 2012). The Raman Effect takes place after electromagnetic radiation interrupts on a particle and intermingled with the electron mass which can be polarized and the bonds of the particle is in the either phase (liquid, solid or gaseous) and the atmosphere in which the particle come across it. For the spontaneous as well as the impulsive Raman effect, which is a type of rigid inelastic light scattering, a photon (electromagnetic waves of a definite wavelength) stimulates (work together with) the particle in whichever the position, Rovibronic Situation (least possible rotational in addition to the vibrational force level of the position electronic situation) or an Agitated Rovibronic Status.
Raman Spectroscopy
As a consequence, in the particle, in a professed virtual vigor state, for a diminutive time period before a rigid and inelastically speckled photon shows result. The inelastically scattered photon which was found as a result and is emitted or scattered might be of any form, i.e. minor (Stokes) otherwise advanced (anti-Stokes) power than the received photon. Spontaneous as well as impulsive Raman scattering is characteristically very feeble, and as a consequence the major complexity of Raman spectroscopy is straightening out the fragile inelastically scattered light from the concentrated Rayleigh scattered laser light. In the past, Raman spectrometers were used holographic gratings as well as numerous scattering stages to attain an elevated degree of rejection of laser. Earlier, the choice of detectors was photomultipliers that were used for dispersive Raman units, long acquisition times came out as a result. On the other hand, recent instrumentation commonly makes use of a mark or edging filters intended for laser rejection along with spectrographs either axial transmissive (AT), FT (Fourier transform spectroscopy based) or Czerny–Turner (CT) monochromator as well as CCD detectors (Levi, Minghetti and Aloisi, 1998).
There are numerous highly developed form of Raman spectroscopy, together with surface-enhanced Raman, tip-enhanced Raman, resonance Raman, polarized Raman, hyper Raman, transmission Raman, stimulated Raman (analogous to stimulated emission) and spatially offset Raman. In Raman scattering spectroscopy the out coming vibronic state of the particle has a different vibrational or rotational than in which the molecule was, initially, before colliding with the upcoming photon (in the form of electromagnetic radiation). The Raman effect is because of inelastic scattering as well as it should not be puzzled with emission (that includes fluorescence or phosphorescence) in which a particle in an excited electronic state give off an energy in the form of photon and came back to the ground electronic state (Boter and Toet, 2010). If the concluding vibrational state of the particle is more energetic or vigorous than the initial state, the inelasticity of the scattered photon will be changed to a lower frequency to maintain the total energy of system balanced. This change in frequency is appointed as a Stokes shift (Yang, Henderson and O'Donnell, 1993). If the last vibrational state energy is of a smaller amount than the initial state, then the inelastically of scattered photon will be changed or shifted to a frequency with higher value, and this is known as an anti-Stokes shift. Raman scattering is an example of inelastic scattering and Rayleigh scattering is an example of elastic scattering (Caronna, Natali and Cupane, 2005).
Forms of Raman spectroscopy
Clinical applications of Raman spectroscopy include the Bio-compatibility, Drug/cell interactions, Metabolic accretions, Cell sorting, Disease diagnosis, Photodynamic therapy (PDT), Bone structure, characterization of bio-molecules, Single cell analysis, DNA/RNA analysis (Kashtan, 2006). Advantages include that it can be used with solids and liquids, not interfered by water, sample preparation is not needed, it is also non-destructive, highly specific for example a chemical fingerprint of a sample. Raman spectra are obtained quickly within seconds, samples can be examined through polymer packaging or glass, Raman scattered light can be transferred over long distances by optical fibers for remote analysis, even for very small volume Raman spectra can be used, Inorganic samples can be analyzed easily by this spectroscopy.
Above applications of both the spectroscopy make them preferred over other spectroscopy for analysis of biomaterials.
Peak at the 1730.71 cm-1 interprets the absorption band of fatty acid ester. The band at 1597.33 cm-1 shows the C=N, NH2 groups in the compound, whereas the peak at 1446.67 cm-1 interprets the CH2 bending made of proteins and the lipids, it also shows protein peak in the sample. The peak at 1367.37 cm-1 demonstrates the C-O stretching, deformation of C-H and deformation of N-H. The peak at 1310.22 cm-1, proves the presence of amide III. The band at 1018.51 interprets the occurrence of ring structure in the sample. Peak at 750.69 cm-1 show out of plane bending of CH2, 6. Based on the above information it is concluded that, sample contains ring structure, C-H, C=N, NH2, C-O, and N-H groups, and the material is Polyurethane.
Peak at the 1664.87 cm-1 shows the occurrence of amide I, C=O group of cytosine, uracil. The band at the 1549.73 cm-1 shows the amide II of proteins is present in sample material. Value 1417.38 cm-1 interprets the stretching of C-N, deformation of C-H and N-H group. Both the value that is 1264.34 cm-1 and 1107.04 cm-1 interprets the occurrence of ring structure. Band at 1243.5 cm-1 interprets the presence of asymmetric phosphate [PO2- (asym.)], amide III of collagen. Band at 875.54 shows the antisymmetric stretch vibration of choline group N+ (CH3) 3. The peak value at 484.09 cm-1 and 1023.19 cm-1 both shows the glycogen occurrence. Based on this interpretation the sample material is supposed to be the compound of hydroxyapatite and polymer.
The peak at 1620.89 cm-1 shows the C=C pophyrin in the sample. Value of 1540.74 cm-1 shows the amide carbonyl group. The peak value at 1446.04 cm-1 interprets the CH2 bending made of proteins and lipids, and the CH2 deformation. Occurrence of ring structure is shown by the peak at 1263.94 cm-1 and the peak at 786.62 cm-1. The band at 1119.27 cm-1 and 868.54 cm-1 shows the C-C stretching. The above information suggested that sample material is Polyurethane of Raman.
The peak value at 717.76 cm-1 shows the presence C-N group (membrane phospholipid head), and the lipids. The band at 583.20 cm-1 interprets the OH- group out of the plane, bending, free. The value 428.59 cm-1 suggested the symmetric stretching vibration of PO43- (phosphate of HA). On the basis of above information it is interpreted that sample D of Raman is bone.
In the Raman spectroscopy chemists glow a laser on a molecule that are in small group and then calculate the amount of light which bounced back. The photons that are the energy packets from the source of light make the molecules vibrate as well as interact with the attachments that grip molecules collectively causing a change or shift into their frequency—it is the type of scattering which results or values is unique for every type of particle and therefore permits for the system or technique to be utilized as a means of classifying the types of molecule.
Recent researches includes that with the help of Surface-Enhanced Raman Spectroscopy (SERS) weak Raman signals can be detected (Kudelski, 2006). One of the main benefits of Surface Enhanced Raman Spectroscopy is that it can be utilized to examine trace amounts of sample material though still having characteristics of non-destructive. It works also in ambient conditions; it has a wider range of wave number as well as it can be utilized in different fields from electrochemistry to materials sciences. Its shell or surface sensitivity makes it to be used for examining and analysis of organic systems including the biological systems. It is helpful also for a various real-life applications, for example, detecting warfare agents of chemicals. It has applications in medical field also that it is being utilized in biosensors to identify biological samples, even including those that are involved in Alzheimer’s disease as well as in cancer (Wood, 2012). It is also being capable to be used in fraud detection. As an example, it identifies dyes in paintings, even at low concentrations and therefore helps to date them as well as it, therefore validate them. Pittcon in this year, the world’s biggest annual conference as well as an exhibition for laboratory science, takes place from the 6th-10th March in Atlanta, Georgia. With remarkable technical program, the occasion featured presentation more than 2000. Along with, topics that included the utilization of Surface Enhanced Raman Spectroscopy for detection of fragile raman signals. HORIBA has established a Raman spectroscopy system and designing as well as manufacturing them for more than four decades.
Research of the capability of Raman spectroscopy for detection of oral cancer in surgical margins. The poor prediction of Oral Cavity Squamous Cell Carcinoma (OCSCC) patients is related with left over tumor even after surgery (Maund and Jefferies, 2015). Raman spectroscopy has the capability to present a purpose of intra-operative assessment of the surgical fringes. The aim was to be aware of the biased basis of Raman spectroscopy at a level of histologically. In whole, 127 false or pseudo-color images of Raman were produced from thin tissue sections that are unstained of 25 samples (14 healthy and 11 OCSCC) out of 10 patients. These descriptions were evidently related to the histopathological evaluation of the similar sections after eosin-staining and hematoxylin staining. In this manner, Raman spectra were explained as surrounding healthy tissue structure (i.e., connective tissue (CT), squamous epithelium, adipose tissue, gland, muscle, or nerve) or as Oral Cavity Squamous Cell Carcinoma (OCSCC). These explained that spectra were utilized as input for LDA (Linear Discriminant Analysis) models to differentiate between healthy tissue spectra and OCSCC spectra. A record was obtained with 632 healthy tissue spectra and 88 Oral Cavity Squamous Cell Carcinoma or OCSCC spectra. The Linear Discriminant Analysis or LDA models could differentiate spectra of OCSCC from the spectra of nerve, gland, connective tissue (CT), adipose tissue, muscle, and squamous epithelium in 97%, 94%, 93%, 100%, 100%, and 75% of the cases, respectively (CUI and JI, 2009). More particularly, the composition and structure that were most frequently confused with OCSCC or Oral Cavity Squamous Cell Carcinoma were basal layers of epithelium, dysplastic epithelium, connective tissue close to OCSCC, inflammation- and capillary-rich CT, and glandular tissue that is close to OCSCC (Cuneo and Castoldi, 2011). Studies show that how well Raman spectroscopy facilitates comparison between adjoining healthy tissue and OCSCC structures. This information supports the growth of strong and trustworthy classification algorithms for an upcoming performance of Raman spectroscopy in clinical practice.
The research in this field is that the gas sensors with Ultra-sensitive fiber-optic are improved by metal and organic materials (Rao, Zhu and Mo, 2006). Most industrial mid-IR (2.5–10μm wavelength) has technologies of gas-sensing that is based on a counter or bench top Fourier-transform IR (FTIR) gas cells and spectrometers. These instruments are large and costly, therefore making them inappropriate for portable or applications of distributed sensing. The telecommunication optical industry has, though, built up minuscule near-IR (NIR, having wavelength 0.8–2.0μm) optoelectronic devices and the optical fibers that are highly and have low cost (An ultrathin film as near-perfect IR absorber, 2013). This has lead to a significant increase of the rate in the progress of NIR sensors. The major confront lies in the reality that maximum number of gases do not have NIR regions with basic vibration bands. Thus the absorption necessarily comes from the association of the number of fundamental bands of vibration, therefore resulting in comparatively lower sensitivity of detection. FTIR (Fourier transform infrared spectrometer) meets fire testing requirements for analysis of toxicity of gas. One of the main dangerous parts of a fire is the gases that are toxic in nature that can occur from the combustion process. To keep life, save and build up safer objects for trains, airplanes, and buildings, it is important to discover and evaluate the gases that are discharged when materials and products are burning.
Landmark systems of FTIR microscopy get significant developments. Agilent Technologies, Incorporation initiated considerable enhancements to its Fourier transform infrared spectrometer (FTIR) Cary 610 and 620 microscopes. These revolutionary Fourier transform infrared spectrometer microscopy systems are proposed for eventual performance, contributing the major field of observation at the maximum spatial resolution in the such a small period of time. The instrument is deliberated for use in a vast range of functions and applications, that includes biomedical materials, food, polymers, forensics, chemical and pharmaceutical. Single detection of molecule of explosives, contaminants or diseases is now possible (Ishii and Yanagida, 2000). A procedure to merge the ultra sensitivity of shell or surface enhanced Raman scattering (SERS) having a slippery surface originated by researchers of Penn State will make it possible to sense single molecules of various numbers of biological and chemical species from solid, liquid or gas samples. This grouping of laser-based spectroscopy and slippery surface will unlock new applications in analytical molecular diagnostics, chemistry, national security and environmental monitoring.
Conclusion
FTIR and Raman Spectroscopy has various advantages. It is easy to identify the biomaterial using these techniques as they are fast, can identify even small concentration of samples and also it is helpful in detecting the contaminants in the sample. There is a vast scope of both the spectroscopy, as in recent researches it is proved that how it helps in a number of fields that include medical, chemical, pharmacy, engineering, geology. With the help of these techniques it is even possible to detect the differences caused by diseases. Number of researches are still in progress to find the new aspects of FTIR and the Raman spectroscopy. The future of FTIR and Raman Spectroscopy is wide in relation to the biomaterials. As various syndromes can be identified by these techniques.
References
An ultrathin film as near-perfect IR absorber. (2013). Phys. Today.
Berthomieu, C. and Hienerwadel, R. (2009). Fourier transform infrared (FTIR) spectroscopy.Photosynth Res, 101(2-3), pp.157-170.
Boter, H. and Toet, H. (2010). Organophosphorus compounds I: The preparation of some aryl phosph(orothiol)ates and aryl phosphon(othiol)ates. Recl. Trav. Chim. Pays-Bas, 84(10), pp.1279-1283.
Caronna, C., Natali, F. and Cupane, A. (2005). Incoherent elastic and quasi-elastic neutron scattering investigation of hemoglobin dynamics. Biophysical Chemistry, 116(3), pp.219-225.
Chukov, V. (2012). Oscillations of a statistical scattering in the Rayleigh limit and the Rayleigh law violation. Ultrasonics, 52(1), pp.5-11.
CUI, Z. and JI, X. (2009). Feature selection based on linear discriminant analysis. Journal of Computer Applications, 29(10), pp.2781-2785.
Cuneo, A. and Castoldi, G. (2011). Mucosa-associated lymphoid tissue (MALT) lymphoma. Atlas of Genetics and Cytogenetics in Oncology and Haematology, (3).
FTIR Applied to Biological Systems. (2007). Chemistry International -- Newsmagazine for IUPAC, 29(4).
Hanley, Q. (2012). Fourier Transforms Simplified: Computing an Infrared Spectrum from an Interferogram. J. Chem. Educ., 89(3), pp.391-396.
Infrared Spectroscopy. (1968). Nature, 218(5136), pp.49-49.
Ishii, Y. and Yanagida, T. (2000). Single Molecule Detection in Life Sciences. Single Molecules, 1(1), pp.5-16.
Jinda, T. (1991). Preparation and properties of highly dyeable aramid fiber. Sen'i Gakkaishi, 47(11), pp.621-624.
Kashtan, H. (2006). Salvage photodynamic therapy. Photodiagnosis and Photodynamic Therapy, 3(1), p.17.
Kudelski, A. (2006). New Trends in the Surface-Enhanced Raman Spectroscopy. ChemInform, 37(2).
Levi, G., Minghetti, L. and Aloisi, F. (1998). Regulation of prostanoid synthesis in microglial cells and effects of prostaglandin E2 on microglial functions. Biochimie, 80(11), pp.899-904.
Maund, I. and Jefferies, S. (2015). Squamous cell carcinoma of the oral cavity, oropharynx and upper oesophagus. Medicine, 43(4), pp.197-201.
NORRISH, R. (1960). Infra-Red Frequency Shifts. Nature, 187(4732), pp.142-142.
Raman Spectroscopy. (2006). Chemistry International -- Newsmagazine for IUPAC, 28(3).
Rao, Y., Zhu, T. and Mo, Q. (2006). Highly sensitive fiber-optic torsion sensor based on an ultra-long-period fiber grating. Optics Communications, 266(1), pp.187-190.
Sánchez-de-la-Llave, D. (2009). Multiple beam Michelson-based interferometer. Optical Engineering, 48(8), p.085601.
Wood, H. (2012). Alzheimer disease: Prostaglandin E2 signalling is implicated in inflammation early in the Alzheimer disease course. Nature Reviews Neurology.
Yang, F., Henderson, B. and O'Donnell, K. (1993). The origin of the Stokes shift. Physica B: Condensed Matter, 185(1-4), pp.362-365.
To export a reference to this article please select a referencing stye below:
My Assignment Help. (2016). FTIR And Raman Spectroscopy For Biomaterials Analysis. Retrieved from https://myassignmenthelp.com/free-samples/ftir-spectroscopy.
"FTIR And Raman Spectroscopy For Biomaterials Analysis." My Assignment Help, 2016, https://myassignmenthelp.com/free-samples/ftir-spectroscopy.
My Assignment Help (2016) FTIR And Raman Spectroscopy For Biomaterials Analysis [Online]. Available from: https://myassignmenthelp.com/free-samples/ftir-spectroscopy
[Accessed 01 June 2025].
My Assignment Help. 'FTIR And Raman Spectroscopy For Biomaterials Analysis' (My Assignment Help, 2016) <https://myassignmenthelp.com/free-samples/ftir-spectroscopy> accessed 01 June 2025.
My Assignment Help. FTIR And Raman Spectroscopy For Biomaterials Analysis [Internet]. My Assignment Help. 2016 [cited 01 June 2025]. Available from: https://myassignmenthelp.com/free-samples/ftir-spectroscopy.
