Overview of Cell Proliferation Assays
Question:
Discuss about the Immunological Tests for Mixed Lymphocyte Reaction.
These tests are mainly cell proliferation assays. They may be of two types which are the one way MLR and the two way MLR. There are many other ways by which the scientists or any other organizations can follow their guidelines for performing the test. One way MLR can be described as the cell proliferation assay where a particular population of lymphocytes (called the responder cells) gets stimulated by another population of lymphocytes called the stimulator cell. After incubation period for about five to six days, Bromodeoxyuridine (BrdU) are mainly mixed and then incorporated into the DNA of the proliferating cells (Brunner & Cerottinii, 2014). They are then incubated there for another day or half. Following this, the BrdU is washed out from the mixture. Then Enzyme-linked Immunosorbant Assay (ELISA) is performed. This helps to quantify the total amount of BrdU which gives a measure of cell proliferation. Actually in the end, the expert is mainly evaluating the proliferation response of the target cells which occur when both the population of lymphocytes are mixed together. In two way MLR tests, there is only one difference. Here, both the populations of the lymphocyte cells are able to proliferate. As a result, the experimental design is set in such a way so that, it can support the proliferation of both lymphocytes. However, the analysis of the amount of the proliferated cells is mainly done by the mixing of two populations of lymphocytes (Hong et al., 2017).
The tests are mainly performed so that proper assessment can be made that whether proliferation of T-cell is increased or had undergone inhibition in response to the external or any other cellular stimuli. An inhibited cell proliferation states the decrease of the T-cell response to the test article. An increase in the cell proliferation indicates an increase in T cell response to the test article. They are mainly used by the biotech and pharmaceutical organizations to understand whether their drug or any other implantable material is safe or not (Konina et al., 2013).
Pokeweed mitogen test is a type of lymphocyte stimulation test which use a substance called mitogen to non specifically activate the lymphoid cells in the sample. Mitogens are derived from plants and can bind with molecules on the surface of the lymphocytes, thereby triggering their activation called blastogenesis. This is followed by subsequent division or proliferation. Pokeweed mitogen test is mainly considered to be a B cell mitogen. At first the lymphocytes are suspended and maintained in tissue culture medium which is supplemented with serum obtained from normal animals of same species as the lymphocytes are being assayed. The lymphocyte cell suspension is then aliquoted into a number of multi-well plates and culture media followed by the adding of mitogenic substance to relevant culture wells. Cultures without the presence of mitogen should be kept for control purposes. The total volume for each of the well should be kept constant and only the concentration of the lymphocytes and the mitogens should be changed form one well to another (Salak et al., 2016). All well on the plate should be set in triplicate style for analysis. The culture periods should vary between 2 to 5 days in a tissue culture incubator at 37 degree Celsius and 5% CO2. Within the last few hours, a set quantity of radio-labelled thymidine should be added to each of the well in the plate but not in the triplicate of well that act as controls. Radio labeled thymidine is then inserted into newly synthesized DNA as the cellular divisions continue in wells. Culture is then stopped, contents of each well are mechanically aspirated, cells are collected in filter mat and all culture fluid, unincorporated thymidine and others are released. The amount of beta radiation emitted is mainly proportional to the total amount of thymiden which is radio-labelled and incorporated in the lymphocytes. This thereby shows how it is proportional to the rate of division. The result is mainly recorded as the stimulation index (SI) which takes into account the level of background cell division that occurs in control well not exposed to mitogen. When such result is greater than 3, it is considered as significant result.
The Pokeweed Mitogen Test
The measurement of the human lymphocyte’s proliferative responses to different types of stimuli can be considered as an important technique which is used to assess their biological functions as well as their status. Mitogens are able to nonspecifically stimulate lymphocytes proliferation which in turn helps to evaluate the immune responsiveness of the patient
This can be defined as the test in which a fluorescent dye is mainly used to stain an antibody so that proper identification of clinical specimens can be conducted. The fluorescent dyes help in conjugation with the immunoglobulin in such a way so that there is no alteration in the antibody-antigen reaction. These make the dyed organism (if present) glow visibly when examined under a fluorescent microscope. Two techniques can be used in this type of tests. The first one is the direct test where the immunoglobulin is first conjugated with a fluorescent dye and then it is added to the tissue (Bizzaro et al., 2014). Following this, it combines with the specific antigen. The other type is called the indirect antibody technique. In this type, the immunoglobulin is first added to the tissue. Then it is made to combine with the specific antigen after which the antigen antibody complex gets labeled with a fluorescent antibody.
This test is mainly helpful in identification of a wide range of infective and other agents for which specific antibodies linked to fluorescein are held. The observation of the fluorescence demonstrates that the antibody has been successful in attaching with the corresponding antigen if present and thereby provided appropriate outcomes (Wang et al., 2014).
The utility can be described in examples. This test can be used in the identification of Mycobacterium tuberculosi. It is also the most common serological test for syphilis. Moreover, it is also seen that when humans are infected by rabies virus, the virus proteins which are the antigens get incorporated in their tissues. As rabies remains present in the nervous tissue, fluorescently-labeled anti-rabies antibody a (Direct fluorescent antibody test) is conducted which helps in identification of the presence or absence of the antigen in the human being (Roberdet et al., 2013).
The aim of the Plaque-forming cell assay is the evaluation of an individual to mount a humoral response or simply an antibody response to a particular antigen typically like that of the sheep red blood cells called the SRBC.
When exposure to the SRBC or any other T-cell dependent antigens takes place, generation of antigen specific antibody response requires bit cooperation as well as interaction of the several immense cell types. These include B cells, T cells and also antigen presenting cells like macrophages. Alteration in the functioning of any such cells can impair the ability of the B cells to produce antigen specific antibody. Due to this complexity of the interaction of different types of cells, quantification of the plaque forming cell response which is actually antibody forming cell response SRBC response was found to be important as the best predictors of immuno-toxicity (Ladics, 2016).
Fluorescent Antibody Techniques
The PFC response to SRBC mainly incorporates the usage of immuno-competetent cells from lymphoid organs mainly like the spleen. In association with this, other antigens known as T cell independent antigens can be used in the PFC assay for identification of the primary immune types of cells which are targeted by particular compound. These antigens do not require the need for the T cells in the eliciting any kinds of production of the antibody by the B cells. In spite of using the antigen, the PFC assay is based on SRBC. Which include the antigen as well as the conjugated hapten for the specific antibody produced by the B cells (Leftkovits & Pernis, 2014).
Spleens from SRBC immunized animals are removed and cells are mixed then with SRBC. It is complemented in SRBC media like agar. The mixture is then placed on petridish, covered and incubated for 3 hours at 37degree Celcius. During incubation, B cells produce IgM antibody which are specific for SRBC. This then bind to SRBC membrane antigens. This is then found to cause complement mediated lysis of the SRBC. Subsequent formation of plaques takes place. These can be counted visually. Data is usually expressed as IgM AFC(PFC)/ spleen
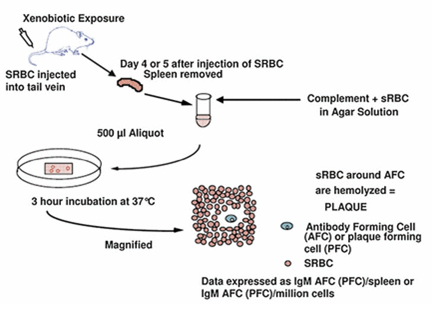
Source: Ladics, 10.1007/978-3-642-27786-3_1171-3
The cytotoxic T lymphocyte response against T-dependent antigens require cooperation of major categories of immune cells which include professional antigen presenting cell like dendritc cells, CD4+ T helper cells and CD8+ T effector lymphocytes. It helps in evaluating antigen presentation via class 1 and class 2 MHC (major histocompatability complex). Therefore, CTL assay is also excellent candidate for the evaluation of potential immunotoxicity in the immune system (Burleson, Burselon & Deitart, 2010).
51Chromium (Cr) Release Assays is one type of assay. This is the classical assay to detect the CTL activity. This assay mainly evaluates the ability of the CD8+ cytotoxic T cells to lyze target cells which expresses an epitope of interest. Target cells are first stimulated with the help of an appropriate peptide to activate the cells. Then the target cells which express the epitope of interest are labeled with 51Cr. Cells are then incubated by CTL effector cells. As the CTL effectors cells are binding to the antigen specific target, these number of cell will go lyses and the 51Cr will be eventually released in the culture supernatant (Xu et al., 2013). The cells are then centrifuged and the levels of 51Cr in the supernatant are measured by scintillation. In the recent years, a variety of similar assays are proposed which do not use radioactive agents. They involve measuring of the CD8+ cytotoxic T cells. These help in determination of levels of CD8+ cytotoxic activity (Mucida et al., 2013). Researchers are of the opinion that that the later are more sensitive to that of the traditional method.
References:
Bizzaro, N., Antico, A., Platzgummer, S., Tonutti, E., Bassetti, D., Pesente, F., ... & Study Group on Autoimmune Diseases of the Italian Society of Laboratory Medicine. (2014). Automated antinuclear immunofluorescence antibody screening: a comparative study of six computer-aided diagnostic systems. Autoimmunity Reviews, 13(3), 292-298.
Brunner, K. T., & Cerottini, J. C. (2014). Cytotoxic lymphocytes as effector cells of cell-mediated immunity. Progress in immunology, 1, 385-398.
Burleson, G. R., Burleson, F. G., & Dietert, R. R. (2010). The cytotoxic T lymphocyte assay for evaluating cell-mediated immune function. Immunotoxicity Testing: Methods and Protocols, 195-205.
Hong, J. W., Lim, J. H., Chung, C. J., Kang, T. J., Kim, T. Y., Kim, Y. S., ... & Lew, D. H. (2017). Immune Tolerance of Human Dental Pulp-Derived Mesenchymal Stem Cells Mediated by CD4+ CD25+ FoxP3+ Regulatory T-Cells and Induced by TGF-β1 and IL-10. Yonsei Medical Journal, 58(5), 1031-1039.
Khonina, N. A., Broitman, E. V., Shevela, E. Y., Pasman, N. M., & Chernykh, E. R. (2013). Mixed lymphocyte reaction blocking factors (MLR-Bf) as potential biomarker for indication and efficacy of paternal lymphocyte immunization in recurrent spontaneous abortion. Archives of gynecology and obstetrics, 288(4), 933-937.
Ladics, G. S. (2016). Plaque-Forming Cell Assays. Encyclopedia of Immunotoxicology, 698-702.
Lefkovits, I., & Pernis, B. (Eds.). (2014). Immunological methods (Vol. 3). Elsevier.
Mucida, D., Husain, M. M., Muroi, S., Van Wijk, F., Shinnakasu, R., Naoe, Y., ... & Attinger, A. (2013). Transcriptional reprogramming of mature CD4+ helper T cells generates distinct MHC class II-restricted cytotoxic T lymphocytes. Nature immunology, 14(3), 281-289.
Robardet, E., Andrieu, S., Rasmussen, T. B., Dobrostana, M., Horton, D. L., Hostnik, P., ... & Servat, A. (2013). Comparative assay of fluorescent antibody test results among twelve European National Reference Laboratories using various anti-rabies conjugates. Journal of virological methods, 191(1), 88-94.
Salak, J. L., McGlone, J. J., Morrow, J. L., Hurst, R. J., & Green, R. D. (2016). Genetic Variability in Measures of Beef Cattle Immune Response. Texas Journal of Agriculture and Natural Resources, 3, 54-56.
Wang, C., Bao, C., Liang, S., Zhang, L., Fu, H., Wang, Y., ... & Ni, J. (2014). HAI-178 antibody-conjugated fluorescent magnetic nanoparticles for targeted imaging and simultaneous therapy of gastric cancer. Nanoscale research letters, 9(1), 274.
Xu, Z., Ramishetti, S., Tseng, Y. C., Guo, S., Wang, Y., & Huang, L. (2013). Multifunctional nanoparticles co-delivering Trp2 peptide and CpG adjuvant induce potent cytotoxic T-lymphocyte response against melanoma and its lung metastasis. Journal of Controlled Release, 172(1), 259-265.
To export a reference to this article please select a referencing stye below:
My Assignment Help. (2018). Cell Proliferation Assays, Pokeweed Mitogen Test, Fluorescent Antibody Techniques, Plaque-forming Cell Assay, CTL Assay. Retrieved from https://myassignmenthelp.com/free-samples/immunological-tests-mixed-lymphocyte-reaction.
"Cell Proliferation Assays, Pokeweed Mitogen Test, Fluorescent Antibody Techniques, Plaque-forming Cell Assay, CTL Assay." My Assignment Help, 2018, https://myassignmenthelp.com/free-samples/immunological-tests-mixed-lymphocyte-reaction.
My Assignment Help (2018) Cell Proliferation Assays, Pokeweed Mitogen Test, Fluorescent Antibody Techniques, Plaque-forming Cell Assay, CTL Assay [Online]. Available from: https://myassignmenthelp.com/free-samples/immunological-tests-mixed-lymphocyte-reaction
[Accessed 25 May 2025].
My Assignment Help. 'Cell Proliferation Assays, Pokeweed Mitogen Test, Fluorescent Antibody Techniques, Plaque-forming Cell Assay, CTL Assay' (My Assignment Help, 2018) <https://myassignmenthelp.com/free-samples/immunological-tests-mixed-lymphocyte-reaction> accessed 25 May 2025.
My Assignment Help. Cell Proliferation Assays, Pokeweed Mitogen Test, Fluorescent Antibody Techniques, Plaque-forming Cell Assay, CTL Assay [Internet]. My Assignment Help. 2018 [cited 25 May 2025]. Available from: https://myassignmenthelp.com/free-samples/immunological-tests-mixed-lymphocyte-reaction.
