History of polymer
Describe about the Polymer for Polychlorotrifluoroethylene.
Berthelot first used the term “polymer” in an article, which is published in “the Bulletin of the Chemical Society of France.” He described it as, “styrolene (styrene), heated at 2000 during a few hours, transforms itself into a resinous polymer.” Therefore, the first synthetic polymer was produced from styrolene. The polychlorotrifluoroethylene (PTFCE or PCTFE) was first discovered in 1934 by Otto Scherer and Fritz Schloffer in Germany (LaBarbera, 2014). This polymer is commercially available in the trade name of Kel-F 81® by M W Kellogg Company. This trade name was derived from Kellogg and Fluoropolymer. The other trade names are Neoflon, Aclon and Aclar. The polychlorotrifluoroethylene (PCTFE) is prepared by the polymerization of chlorotrifluoroethylene (monomer). Although, polychlorotrifluoroethylene still is commercially known as Kel-F®, 3M stopped manufacturing Kel-F® PCTFE in the year of 1995. The right of this polymer was sold to the Daikin who manufactures the same product under the brand name of NEOFLON™ PCTFE. The PCTFE is recognized as a crystalline polymer whose type of crystallinity and degree can be controlled by controlling its thermal history. Therefore, depending on the manufacturing conditions the properties of PCTFE can be changed. When PCTFE is molded with high crystalinity, it is found that PCTFE become dense and consists of high mechanical strength but low elongation. On the other hand, amorphous molded PCTFE consists of lower density and is more elastic as well as optically clear. It is also recognized that PCTFE objects, which are prepared by compression molding are found to be stronger than those objects that are prepared by injecting extrusion or molding.
Fritz Schloffer and Otto Scherer in Germany first discovered the polychlorotrifluoroethylene polymer in 1934. These scientists worked under IG Farben Company in Germany. However, after World War II M W Kellog Company (Millett et al., 2016) commercialized this polymer under the trade name of Kel-F 81 in 1950s. After 1957, the M W Kellogg company was taken by the 3M Company and they stopped producing polychlorotrifluoroethylene. However, polychlorotrifluoroethylene is now manufactured in different trade names such as Neoflon (from Dalkin), Aclon (Allied Signal) and Aclar (Allied signal). Apart from these other trade names of this product are Hostaflon C2, Voltale (Arkema), Fluon (ICI) and Plaskon (Allied Chemical Corporation).
The polychlorotrifluoroethylene is formed by the polymerization of the chlorotrifluoroethylene. The molecular formula of the polychlorotrifluoroethylene is (C2CIF3)n. in this formula the n represents number of monomers (chlorotrifluoroethylene) in the polymer molecule (polychlorotrifluoroethylene). The monomer of the polychlorotrifluoroethylene consists of two carbons, three-fluorine and one-chlorine atoms (Wypych, 2016).
Origin, Structure and synthesis
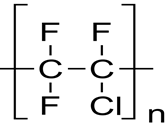
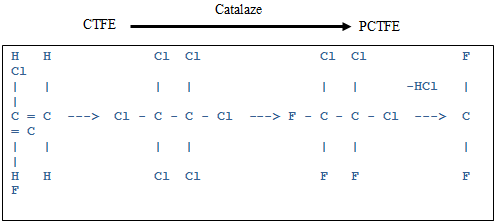
The polychlorotrifluoroethylene (PCTFE) is prepared from the emulsion or aqueous suspension of chlorotrifluoroethylene in the presence of polymerization catalyst, which is similar to teflon. The PCTFE can be prepared through simple free radical polymerization of chlorotrifluoroethylene (CTFE), since polychlorotrifluoroethylene is an “addition homopolymer” (Lopez et al., 2015). Emulsion polymerization is used to produce polychlorotrifluoroethylene, a polymer with high molecular weight. The reaction takes place within 0-400 C. The initiation of the reaction is carried out by the activation of redox systems by a metal salt (persulfate-bisulphit with a ferrous salt).
The industry of PCTFE or polychlorotrifluoroethylene is considered as significant due to the contribution or use of the polychlorotrifluoroethylene in society. More than one type of products are produced from polychlorotrifluoroethylene and used in the society. According to Ebnesajjad (2014), it is found that to keep pace with the globalization the use of PCTFE is valuable. It is found that the production of the PCTFE can also have a direct impact on the economy of a country because of its demand in world market (Ebnesajjad, 2014). According to survey report it is established that North America still dominates the market of fluoropolymers especially PCTFE due to its demand in the United States. Today fluoropolymers especially PCTFE are used in the society in different forms and becomes a valuable part of the human life. The PCTFE is used hugely in medical sciences. Many types of equipment are built with this polymer due to its high tensile strength as well as good thermal characteristics. On the other hand, PCTFE is also useful in ligament replacement, cardiovascular grafts as well as heart patches. In other words the use of PCTFE in medical science changed the lives of many people. Today people are using plugs, bearing, electrical insulation, wire and cables which are prepared with PCTFE. The melting temperature of PCTFE is too high (210- 2150 C), therefore, it is used in packaging and sealing. It is also hugely used in Barrier film and insulation (Firdaus et al., 2015). Therefore, it can be said that the use of PCTFE had change the lifestyle of the common people hugely.
Polychlorotrifluoroethylene has many advantages, which are listed below (Couture et al., 2015):
They are completely transparent.
They have high mechanical strength. They are resistant to stress-crack.
Their low shrinkage property at low temperature provides excrement stability to make valve seats.
They are excellent chemically resistant to all corrosive inorganic liquids.
Importance to society
They have zero moisture absorption.
They have excellent electrical properties, which are maintained in high humidity.
They are also very stiff in nature.
They are resistant to weather.
They are physiologically inert.
Their service temperature varies from -270°C to 205°C.
Their Limiting Oxygen Index is >95 (the best of any plastic material).
polychlorotrifluoroethylene slightly swells into halocarbon compounds, like, aromatic solvents, esters and ethers. Though it is chemically resistant, still it is less than Polytetrafluoroethylene. There are other disadvantages. They have limited mechanical properties. Their cost is very high. They are very difficult to process. They are not decomposed easily. Their decomposition gives rise to toxic products (Ozaki et al., 2014).
The use of PCTFE is versatile. The PCTFE could be used in different industries due to its specific as well as unique characteristics. The use of PCTFE in a versatile way (in industries) started in 1953. The melting temperature of PCTFE is 210- 2150 C and Tensile Modulus is 60-100 MPa, which makes it more flexible. Due to these properties the industries use this polymer in different way (LaBarbera, 2014). The PCTFE is used in different industries including automotive industries, Chemical industry, Electrical industry, architectural as well as in domestic industries, Medical industries even in engineering industries (Goff et al., 2015). In Automotive industries the PCTFE is used in the form of gaskets, shaft seals, power steering, O- rings and many other parts. In Chemical industries the PCTFE could be used in chemical resistance, weather stability and thermal property as coatings for heat exchange, autoclaves and all kind of reaction vessels. In architectural and domestic industries the PCTFE is used to prepare water- repellent fabric, non- stick coatings for cookware, architectural fabric and fiber glass composition. Apart from this in medical industries, the PCTFE is also in various forms such as in ligament replacement, heart patches and cardiovascular grafts (Firdaus et al., 2015). Apart from these, the PCTFE can be used in different medical functions such as surface energy, mechanical property, and biological stability as well as in chemical stability. On the other hand, it is also found that the PCTFE can be used in flame retardancy, barrier film, and packaging and sealing.
The synthetic polymers have large impact on the environment. These are described below:
The synthetic polymers give rise to environmental pollution. One of the common environmental pollution is 44 percent of the seabirds species are recognized to have ingested synthetic polymers. Mistakenly, those birds have used them as food. According to the U.S. National Institutes of Health, due to this ingestion, millions of birds are dying each year (Goff et al., 2015). The death of these shore birds gives rise to significant environmental problem, as these seabirds play a major role to maintain the ecological balance between the population sizes of crustaceans and fish.
Advantages
POPs are known as persistent organic pollutants. They are certain toxins, which stay in the environment without any changes for years, for example, pesticides, like, toxaphene and DDT. The researchers of the University of the Pacific had conducted one research. In that research, they found the synthetic polymers in the sample, collected from the coastal sites of the northern Pacific Ocean (Comiskey & Jacobson, 2013). After research, Harmful toxins are found in that synthetic polymer sample. These synthetic polymers continuously give rise to hazardous chemicals into wildlife and chemicals. It not only misbalances the ocean life but also the human life is misbalanced, when the humans ingest these fishes.
These synthetic polymers are not only polluting the oceans but also environmental problems arise due to the production process. According to the Environmental Working Group organization, DuPont chemical company has exposed contaminants into local watersheds during Teflon production for decades. U.S. Environmental Protection Agency states that these chemicals are accumulated in the fish gills, which are affecting high quantities in the food chain (Soper et at., 2013).
The presence of synthetic polymers is not affecting water and oceans by their productions, but these polymers are also polluting the lands. These polymers will be disposed in landfills. Without any changes, they will stay for centuries. As the time will be passed away, toxins will be released from these into the soil. Clean Air Council organization has stated that each year, only Americans use almost 102.1 billion plastic bags, which are synthetic polymer (LaBarbera, 2014). Among these bags, less than one percent can be recycled. These synthetic polymers not only dispose hazardous chemicals to the soil, but also their and non-biodegradability and longevity properties give rise to constant landfills as the use of these polymers grows and continues.
References:
Comiskey, B., & Jacobson, J. M. (2013). U.S. Patent Application No. 14/088,552.
Couture, G., Ladmiral, V., & Améduri, B. (2015). Methods to prepare quaternary ammonium groups-containing alternating poly (chlorotrifluoroethylene-alt-vinyl ether) copolymers. RSC Advances, 5(14), 10243-10253.
Ebnesajjad, S. (2014). Fluoroplastics, Volume 1: Non-Melt Processible Fluoropolymers-The Definitive User's Guide and Data Book (Vol. 1). Elsevier.
Firdaus, S., Srivastava, S., Ahmad, M., Ansari, S. U. I., & Ali, P. (2015). Poly (chlorotrifluoroethylene): Normal Modes, Phonon Dispersion and Heat Capacity. International Journal, 3(5), 1071-1077.
Goff, M., Hazell, P. J., Appleby-Thomas, G. J., Wood, D. C., Stennett, C., & Taylor, P. (2015). Gas gun ramp loading of Kel-F 81 targets using a ceramic graded areal density flyer system. International Journal of Impact Engineering, 80, 152-161.
LaBarbera, D. A. (2014). Microstructural Modeling of Hot Spot and Failure Mechanisms in RDX Energetic Aggregates. North Carolina State University.
Lopez, G., Gao, C., Li, L., Wyzgoski, F. J., Thenappan, A., Rinaldi, P. L., & Ameduri, B. (2015). Synthesis and microstructural characterization of poly (chlorotrifluoroethylene-co-vinylidene chloride) copolymers. Polymer Chemistry, 6(20), 3790-3799.
Mann, C. A., Marsh, D. G., Hamilton, R. T., Miller, C. E., & Shah, H. (2014).U.S. Patent No. 8,715,463. Washington, DC: U.S. Patent and Trademark Office.
Millett, J. C., Lowe, M. R., Appleby-Thomas, G., & Roberts, A. (2016). The Mechanical and Optical Response of Polychlorotrifluoroethylene to One-Dimensional Shock Loading. Metallurgical and Materials Transactions A,47(2), 697-705.
Ozaki, H., Kawasaki, K., Higuchi, T., & Araki, T. (2014). U.S. Patent Application No. 14/332,151.
Soper, A. K., Page, K., & Llobet, A. (2013). Empirical potential structure refinement of semi-crystalline polymer systems: polytetrafluoroethylene and polychlorotrifluoroethylene. Journal of Physics: Condensed Matter, 25(45), 454219.
Wypych, G. (2016). Handbook of polymers. Elsevier.
To export a reference to this article please select a referencing stye below:
My Assignment Help. (2017). Polymer For Polychlorotrifluoroethylene. Retrieved from https://myassignmenthelp.com/free-samples/polymers-history-of-polymers.
"Polymer For Polychlorotrifluoroethylene." My Assignment Help, 2017, https://myassignmenthelp.com/free-samples/polymers-history-of-polymers.
My Assignment Help (2017) Polymer For Polychlorotrifluoroethylene [Online]. Available from: https://myassignmenthelp.com/free-samples/polymers-history-of-polymers
[Accessed 22 May 2025].
My Assignment Help. 'Polymer For Polychlorotrifluoroethylene' (My Assignment Help, 2017) <https://myassignmenthelp.com/free-samples/polymers-history-of-polymers> accessed 22 May 2025.
My Assignment Help. Polymer For Polychlorotrifluoroethylene [Internet]. My Assignment Help. 2017 [cited 22 May 2025]. Available from: https://myassignmenthelp.com/free-samples/polymers-history-of-polymers.
